The pH of an engineered soil is one of the most common topics for discussion when working on a new project, particularly when pH does not match the project specifications exactly. Because pH is critical to soil health and plant health, it is important to understand what pH is. Simply put, pH is the measure of the concentration of positively charged hydrogen ions in a substance, with a value of 7.0 showing neutrality, higher numbers being more alkaline and lower numbers being more acidic. Because pH is measured on an inverse logarithmic scale, rather than a linear scale, each decrease of one unit is a ten-fold increase in the number of hydrogen ions.
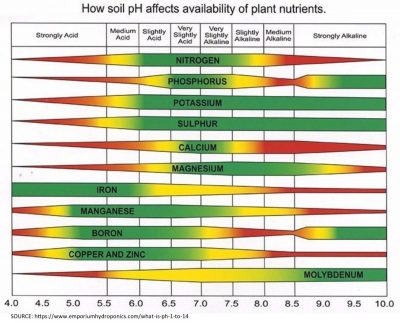
In soil science, pH is an important consideration as it relates to plant health and nutrient uptake. Certain nutrients are available to plants depending on the pH range of the soil, so it is important to consider pH when designing an engineered soil for planters, green roof terraces, landscaping, and other applications. However, most scientific research into soil pH is based on agriculture and crop production, which may not apply in these situations.
Soil pH is a transitory property, especially in the Northeast or any other temperate climate where soil temperatures can change dramatically throughout the year. If soil temperatures are below 50°F, soil biology slows down and pH increases as a result. Soil moisture plays a critical role as well. As soils dry out, pH trends up. Likewise, when water is re-introduced to the soil, either naturally or through watering, the soil pH trends back down. As a result, soil pH should be monitored over time to get an accurate understanding of this dynamic soil characteristic. Over time, soils develop a balance where the influences from moisture level, biology, groundwater, and subsoils produce a natural range of pH values. Changes in pH are expected throughout the year as conditions change.
Construction projects often need to import soils to repair and restore a site after building work is complete. Regulations and common sense do not allow large-scale soil harvesting from construction sites or field locations, which makes finding native soils difficult. Quarries and mines are a reliable source of natural deposits of soils, sand, and other sandy materials, and are often included as components in an engineered soil blend. Because these materials have been buried for thousands of years, they may have accumulated calcium deposits, and when initially excavated, these materials have a naturally high pH. However, this can change rapidly. Once these ancient materials are flushed by rain or irrigation, the pH adjusts quickly.
When designing manufactured soils, it is important to consider pH ranges common to the region. Soils in the Boston area may have a slightly acidic natural pH in the range of 6.0 to 6.8, while upland soils elsewhere in Massachusetts or in New York’s Hudson Valley tend to be slightly alkaline, sometimes with a pH as high as 7.5 or more. It is important to align the characteristics of an engineered soil with the natural soil conditions of the region to best support native plant growth in that climate. Sometimes, project specifications will call for a neutral or slightly acidic soil in a region where such soils do not occur naturally. If an engineered soil is designed to be representative of native soils in the region, the pH of the engineered soil may be higher than what is called for in the project specifications. Once established, the soil pH will buffer and reach a balanced range based on the local conditions.
Soil pH is a complex issue, and it is important to keep local conditions in mind when specifying soil pH for a project design. The trend of measured soil pH over time is more important than a one-off test as soil pH adjusts to local conditions over time. Naturcycle is always glad to discuss this topic in greater detail and work with all parties to optimize soil characteristics and plant health, using nature as our guide.